Reminder: Pharmboy and Ilene are available to chat with Members, comments are found below each post.
Designer proteins that package genetic material could help deliver gene therapy
Courtesy of Ian Haydon, University of Washington

Delivering genetic material is a key challenge in gene therapy. Invitation image created by Kstudio, CC BY
If you’ve ever bought a new iPhone, you’ve experienced good packaging.
The way the lid slowly separates from the box. The pull tab that helps you remove the device. Even the texture of the paper inserts matters to Apple. Every aspect of iPhone packaging has been meticulously designed for a pleasing aesthetic experience.
When it comes to genome editing, good packaging is even more crucial.
In a recent article in the journal Nature, a team of bioengineers here at the University of Washington describe a new type of packaging built to protect genetic material, specifically RNA. This designer packaging consists of proteins which self-assemble into soccer ball-like nanostructures known as capsids. These tiny particles encapsulate RNA, allowing it to move around the bodies of mice for hours without being degraded — sidestepping one of the biggest challenges to successful gene editing.
Delivering genetic material
Moving genetic material (DNA or RNA) throughout the body – or targeting it into specific organs and tissues – is a key challenge in human genome editing. In addition to technology like CRISPR, which physically cuts DNA, some potentially lifesaving gene therapies will require the insertion of new genetic elements to serve as templates for repair. But these genetic blueprints face perilous conditions once they enter the body.
Because deadly infections often start when unwanted genetic material from a pathogen makes it into our cells, our bodies have evolved sophisticated ways of quickly detecting and demolishing foreign DNA and RNA molecules. Simply put: Unprotected genetic material doesn’t stick around for very long. In fact, CRISPR itself evolved in bacteria to perform precisely this search-and-destroy function before it was co-opted by scientists as a gene-editing tool.
Biotechnologists have known about this delivery problem for some time. Most researchers have turned to what might sound like a surprising solution: engineered viruses.
Viruses contain their own genetic material which they insert or inject to infect a cell. If viruses can be redesigned to instead transmit human-specified genetic material into the cells of patients without also making them sick, the thinking goes, then perhaps they could serve as the physical packaging for new therapeutic bits of DNA or RNA.
Current gene therapy trial participants are injected with billions of copies of a corrective gene encased in a modified virus. AP Photo/Eric Risberg
The most popular virus for delivering molecules into human cells at present is the adeno-associated virus, or AAV. Not only is this virus a darling of laboratory research, the Food and Drug Administration is poised to approve a pioneering gene therapy which employs it after recent clinical trials revealed engineered AAVs could help safely restore limited sight to the blind. But, experts note, this benign virus is not a perfect solution to the gene delivery problem.
A virus-free solution
Using a repurposed virus to deliver a custom genetic payload is a bit like using a repurposed box to deliver a new iPhone. It can work, but it may not give the best results. The goods can arrive damaged or not at all, and repurposed viruses can also inflame the immune system. Researchers are still trying to figure out how to tweak them so they behave in safe and predictable ways.
Rather than starting with a complex, difficult-to-modify virus, my colleagues here at the Institute for Protein Design began their work with a relatively simple designer protein capsid. This empty vessel did not yet hold any RNA.
The team used computer-guided protein design and artificial laboratory evolution to create a suitable encapsulating structure. They were able to produce one nanostructure that engulfs RNA blueprints at a rate comparable to the best engineered AAVs.
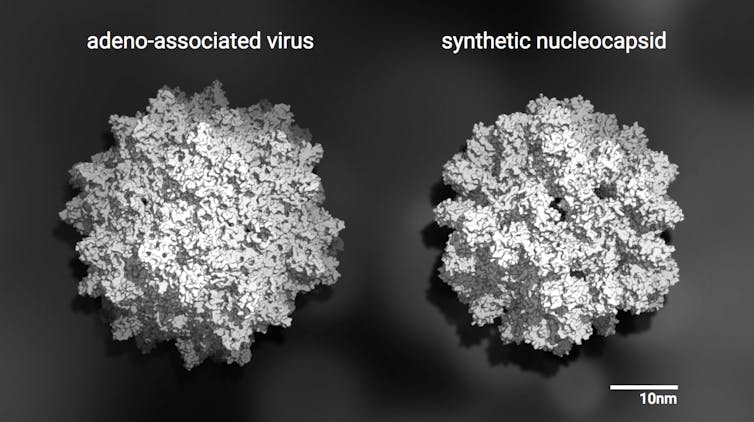
Both of these tiny, soccer ball-like structures package genetic material. On the left, a natural virus. On the right, a computer-generated capsid (which cannot replicate). A thousand billion billion copies of either one could fit inside a real soccer ball. Ian Haydon, CC BY-ND
To begin, they modified the interior surface of a computer-designed capsid so that RNA could stick to it. This got some genetic material inside but didn’t afford it much protection. By mutating this version of the capsid in the laboratory and picking out the best performing mutants, they were able to hone in on new versions which packaged even more RNA, protected it, and persisted inside mouse blood (a hostile environment for foreign RNA and proteins).
In other words, the team made use of one of nature’s favorite strategies: evolution.
“We were surprised it worked so well, to be honest,” said Gabe Butterfield, a lead author of the study. “Evolution was able to hit upon a small number of mutations that made large improvements in complex properties [like persisting in mouse blood].”
Toward gene therapy
Marc Lajoie, another lead author, is optimistic about the future of these designer capsids, but thinks they are “pretty far away” from use in patients.
“We certainly have plenty of work ahead of us,” said Lajoie. But with this two-pronged approach that combines viruses’ capacity to evolve with modern biotech’s abilities to design synthetic nanomaterials, they have their long-term sights set on engineering molecules that “deliver diverse cargos [ranging] from small molecule drugs to nucleic acids to proteins” within human bodies.
![]() With smartphones, well-designed packaging plays a supporting aesthetic role. But if gene therapy is to become a fixture of medicine in the 21st century, innovative packaging may be essential.
With smartphones, well-designed packaging plays a supporting aesthetic role. But if gene therapy is to become a fixture of medicine in the 21st century, innovative packaging may be essential.
Ian Haydon, Doctoral Student in Biochemistry, University of Washington
This article was originally published on The Conversation. Read the original article.