Rapid COVID-19 tests can be useful – but there are far too few to put a dent in the pandemic

Rapid tests for COVID-19 are easy to administer and give fast results. AP Photo/Julio Cortez, File
Courtesy of Bonnie LaFleur, University of Arizona and Katherine Ellingson, University of Arizona
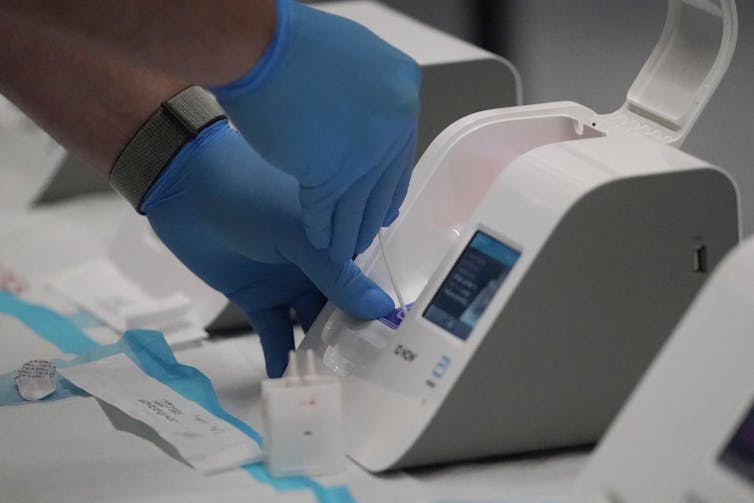
Since September, the Food and Drug Administration has approved seven COVID-19 tests that yield results in 30 minutes or less, offering hope for vast improvements in test access and efficiency throughout the U.S. Most of these are antigen tests that look for viral proteins and can be processed on portable machines or cards.
The idea behind these rapid tests is to detect symptomatic, pre-symptomatic and asymptomatic infectious people before they can spread the coronavirus. But despite massive distribution of these tests by federal officials – including to date over 40 million of 150 million rapid tests ordered from the medical company Abbott – COVID-19 transmission has been surging in every state since early November.
This calls into question whether the current influx of rapid tests can actually slow the spread of COVID-19.
In some targeted applications – and if people take other precautions including mask wearing and social distancing – rapid tests can be a valuable tool. But the current state of availability and accuracy of these tests greatly limit how effective they are at slowing the spread of the virus in communities.
Rapid tests like the Abbott ID Now require only small, portable machines and can give results in 15 minutes, but they sacrifice accuracy. AP Photo/Jeff Chiu
Speed over accuracy
Rapid antigen tests are an attractive option because in addition to their speed, they are cheap and easy to produce and therefore more broadly available than the more commonly used gold-standard PCR tests in theory. But these attributes come with a trade-off: less diagnostic accuracy. This makes them an excellent candidate for use as a screening tool, though less useful for accurately diagnosing SARS-CoV-2 infection.
One-time testing does not mean that a person can safely travel or mingle without precautions. And while no test is perfectly accurate, there are real questions about the performance of the new rapid tests.
A few test manufacturers reported accuracy between 84.0% and 97.6% in individuals who are tested within five days after developing COVID-19 symptoms. There is, however, an apparent gap between the reported performance of these tests and what is achieved in the real world. Anecdotally, these tests seem to miss recent, mild and asymptomatic infections – in fact, rapid tests are authorized by the U.S. Centers for Disease Control and Prevention only for use in symptomatic COVID-19 patients. And of course, people can still be infected soon after getting tested.
For rapid tests to effectively limit spread of the coronavirus, experts suggest that they must be conducted with high frequency – you might miss some cases, but if everyone were getting tested all the time, you would catch a lot of cases too. But even frequent testing is not a panacea. It’s only one part of an approach that must also include social distancing, mask wearing and other precautions.
A highly publicized example of how a rapid testing strategy can go wrong occurred when President Trump and many in his inner circle contracted COVID-19, likely stemming from a single superspreading event. Everyone was reportedly getting daily rapid tests, but they were largely ignoring other measures like face masks and social distancing. It is likely that someone was infected and asymptomatic, tested negative, and then started the outbreak.
Widespread, repeat testing
Detecting pre-symptomatic and asymptomatic individuals who are infectious is critical to controlling the coronavirus. Rapid tests can do this, but only if people are screened repeatedly on a schedule – much as what has been happening in some professional and intercollegiate sports.
The idea is that by testing people early and often – perhaps even as much as every day – rapid tests can catch infected people before they spread the coronavirus to others. But on a national scale, that is a huge number of tests.
Researchers have estimated that the U.S. would need to perform at least 20 million rapid tests per day to drive down infections. The 150 million rapid tests ordered by the government in late August were earmarked for high-risk populations, but would barely cover one week for the population at large. And don’t forget that logistic capabilities, compliance to frequent testing and the infrastructure to act quickly on results all need to happen as well.
Nursing homes were some of the first places to receive rapid tests. AP Photo/Ted S. Warren
A targeted approach
There simply aren’t enough rapid tests being produced for the general public to get repeat testing, so the federal government has prioritized deployment of rapid tests to the high-risk population of nursing homes. Federal guidelines for rapid test use in long-term care facilities are a great example of what a testing program might look like – but also illustrate the current challenges in the use of rapid tests.
If even one person in a nursing home tests positive, all staff and residents must be tested every three to seven days until the facility has been free of COVID–19 for 14 days. When a facility has no cases, all staff are required to get tested according to their county’s test-positivity rate – the higher the rate, the more testing is needed.
Yet nursing homes have had problems with accuracy, staffing and costs while using these tests and find themselves again in crisis during the current surge. While the rapid tests certainly helped catch many cases and should be used in these settings, they cannot single-handedly overcome larger issues that contribute to spread in these settings.
Rapid tests can be effective in highly controlled settings where people are tested frequently and other mitigation measures are in place. Look to the success of the NBA bubble as proof. But in other settings where isolation, mask wearing and social distancing are hard to implement or not followed – like nursing homes or the White House – rapid tests have not kept the virus at bay.
Current testing capacity is nowhere near the hundreds of millions of tests per week required to protect the general population. To date, the promise of cheap and convenient COVID-19 tests being the sole means of controlling disease transmission has not been realized.![]()
Bonnie LaFleur, Professor of Biostatistics, University of Arizona and Katherine Ellingson, Assistant Professor of Epidemiology and Biostatistics, University of Arizona
This article is republished from The Conversation under a Creative Commons license. Read the original article.
[The Conversation’s science, health and technology editors pick their favorite stories. Weekly on Wednesdays.]