A liver disease drug could be repurposed to protect against COVID – new research
By Teresa Brevini, University of Cambridge and Fotios Sampaziotis, University of Cambridge
Almost three years into the pandemic, we’re still regularly seeing hundreds of thousands of new COVID cases recorded each day worldwide. In a new study, involving a combination of miniature organ models, donor organs, animals and humans, we’ve shown that a drug used to treat liver disease could be repurposed to protect against COVID-19.
Vaccines are one of the most potent weapons in our pandemic response, but not everyone can benefit from them. COVID vaccines work by training our immune system to recognise and destroy SARS-CoV-2, the virus that causes COVID-19. As such, they’re not effective for people with a poorly functioning immune system, for example patients taking medications to suppress immune function after an organ transplant.
The virus can also disguise itself to avoid the immune system recognising it, by mutating into new variants and thereby lowering vaccine effectiveness.
Finally, vaccines are not equally accessible, with only one in four people in low income countries having received at least one dose.
In light of these challenges, we wanted to develop a strategy to protect from COVID-19 which could complement vaccination. We decided to target the “doorway” that SARS-CoV-2 uses to infect cells, a receptor called ACE2.
The ‘doorway’ to SARS-CoV-2 infection
There are a couple of key reasons we targeted ACE2 receptors. First, blocking this viral entry doorway doesn’t require an optimally functioning immune system, so this method should be effective even in people who are immunocompromised.
And second, ACE2 receptors are produced by our own cells, so are not affected by changes in the virus (that is, new variants), hopefully making this method more resilient as SARS-CoV-2 evolves.
So we were optimistic when we identified an existing drug that could modify ACE2 receptors. It’s possible this drug could be rapidly repurposed against COVID-19.
This research began from a serendipitous finding. In the Sampaziotis lab at the University of Cambridge we focus on liver regeneration and bile duct diseases, which are the leading cause of liver transplantation in children.
Bile is a digestive fluid produced by the liver and drained into the intestine through tubes called bile ducts. At the beginning of the pandemic, we were studying the effects of bile on bile ducts using miniature versions grown in a dish, known as organoids.

We found that a bile-sensing molecule called FXR, which is abundant in the liver, controls the expression of many molecules in bile duct cells, including ACE2. When ACE2 was identified as the viral entry doorway for SARS-CoV-2 we decided to explore whether drugs targeting FXR could reduce ACE2 receptors and therefore viral infection.
We identified that ursodeoxycholic acid (UDCA), a clinically approved drug currently used for liver disease, had this effect on the mini bile ducts. We successfully repeated our experiments using miniature lungs and miniature guts in the lab, as these organs are commonly affected by COVID-19.
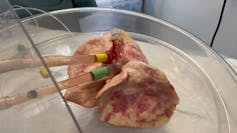
We then validated these findings in hamsters to make sure our lab results held true in a living organism. To test if these findings could be translated to humans, we used a pair of donated human lungs which were not suitable for transplantation. We infected both lungs with SARS-CoV-2, but only one lung was treated with UDCA. We found that the lung that received the drug did not become infected, while the other lung did.
The next step was to test UDCA’s efficacy in reducing ACE2 receptors in humans. We recruited eight healthy volunteers, gave them UDCA, and then swabbed their noses. We observed a reduction of ACE2 in their nasal cells, the main point of entry for the virus into the body, suggesting SARS-CoV-2 would have fewer opportunities to infect these cells.
Finally, since UDCA is widely used in clinical practice, we examined existing data to compare COVID outcomes among people taking UDCA for their liver conditions with outcomes among people not taking UDCA. We found that people taking UDCA were less likely to develop moderate, severe or critical COVID than those who did not receive the drug.
What could this all mean?
UDCA has been on the market for 30 years, and is very safe, with few side effects. In addition, the drug is off-patent, inexpensive, and easy to manufacture, store and administer (it’s taken in tablet form), rendering it convenient to deploy during an outbreak.
Although our results suggest that UDCA could protect against COVID, this study is not a clinical trial and only offers data supporting this hypothesis. The next step will be to confirm our findings in a large randomised clinical trial. We don’t support the use of UDCA for COVID until appropriate policy based on robust clinical evidence is available.
In the future, UDCA would not replace current COVID treatments or highly effective vaccinations, but may be able to expand our arsenal of weapons against the virus. It could offer an alternative strategy which is not dependent on the immune system or subject to immune escape because of viral mutations.![]()
Teresa Brevini, PhD candidate, Wellcome-MRC Cambridge Stem Cell Institute, University of Cambridge and Fotios Sampaziotis, UKRI Future Leaders Fellow, Wellcome-MRC Cambridge Stem Cell Institute, University of Cambridge
This article is republished from The Conversation under a Creative Commons license. Read the original article.