Your immune system makes its own antiviral drug − and it’s likely one of the most ancient
By Neil Marsh, University of Michigan
Antiviral drugs are generally considered to be a 20th century invention. But recent research has uncovered an unexpected facet to your immune system: It can synthesize its own antiviral molecules in response to viral infections.
My laboratory studies a protein that makes these natural antiviral molecules. Far from a modern human invention, nature evolved cells to make their own “drugs” as the earliest defense against viruses.
How antivirals work
Viruses have no independent life cycle – they are completely dependent on the cells they infect to supply all the chemical building blocks needed to replicate themselves. Once inside a cell, the virus hijacks its machinery and turns it into a factory to make hundreds of new viruses.
Antiviral drugs are molecules that inactivate proteins essential to the functioning of the virus by exploiting the fundamental differences in the way that cells and viruses replicate.
One key difference between cells and most viruses is how they store their genetic information. All cells use DNA to store their genetic information. DNA is a long, chainlike molecule built from four different chemical building blocks, each representing a different “letter” of the genetic code. These building blocks are connected by chemical bonds in a head-to-tail fashion to produce strings of millions of letters. The order of these letters spells out the genetic blueprint for building a new cell.
Many viruses, however, store their genetic information using RNA. RNA is built from a chain of four chemical letters, just like DNA, but the letters have slightly different molecular structures. RNA is single-stranded, while DNA is double-stranded. Viral genomes are also much smaller than cellular genomes, typically only a few thousand letters long.
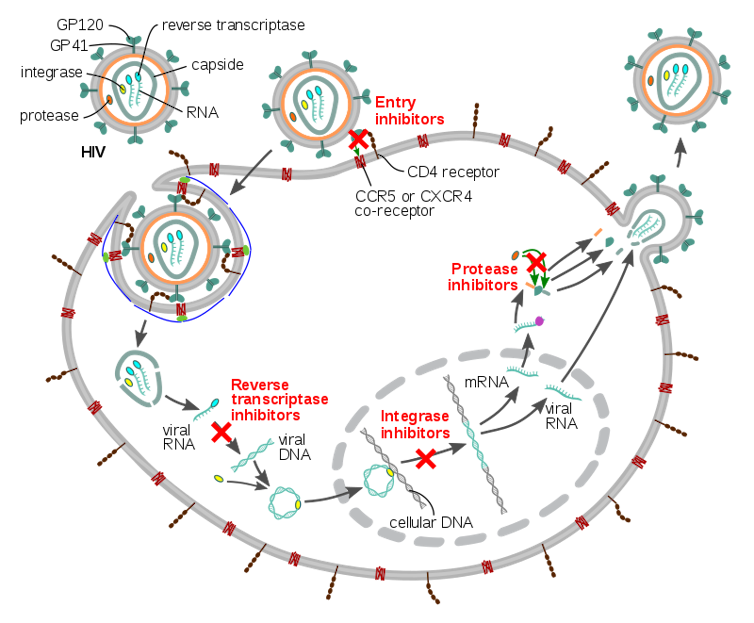
When a virus replicates, it makes many copies of its RNA genome using a protein called RNA polymerase. The polymerase starts at one end of the existing RNA chain and “reads” the string of chemical letters one at a time, selecting the appropriate building block and adding it to the growing strand of RNA. This process is repeated until the entire sequence of letters has been copied to form a new RNA chain.
One class of antiviral drugs interferes with the RNA copying process in a cunning way. The head-to-tail construction of the RNA chain requires each chemical letter to have two connection points – a head to connect to the previous letter and a tail to allow the following letter to be added on. These antivirals mimic one of the chemical letters but crucially lack the tail connection point. If the RNA polymerase mistakes the drug for the intended chemical letter and adds it to the growing RNA chain, the copying process stops because there is nothing to attach the next letter to. For this reason, this type of antiviral drug is called a chain-terminating inhibitor.
Viperin as antiviral producer
Previously, researchers thought that chain-terminating antiviral drugs were strictly a product of human ingenuity, developed from advances in scientific understanding of viral replication. However, the discovery that a protein in your cells named viperin synthesizes a natural chain-terminating antiviral has revealed a new side of your immune system.
Viperin works by chemically removing the tail connection point from one of the four RNA building blocks of a virus’s genome. This converts the building block into a chain-terminating antiviral drug.
This strategy has proved to be highly effective for treating viral infections. For example, the COVID-19 antiviral remdesivir works in this way. A viral RNA polymerase has to join together many thousands of letters to copy a virus’s genome, but an antiviral drug has to fool it only once to derail its copying. An incomplete genome lacks the necessary instructions to make a new virus and becomes useless.
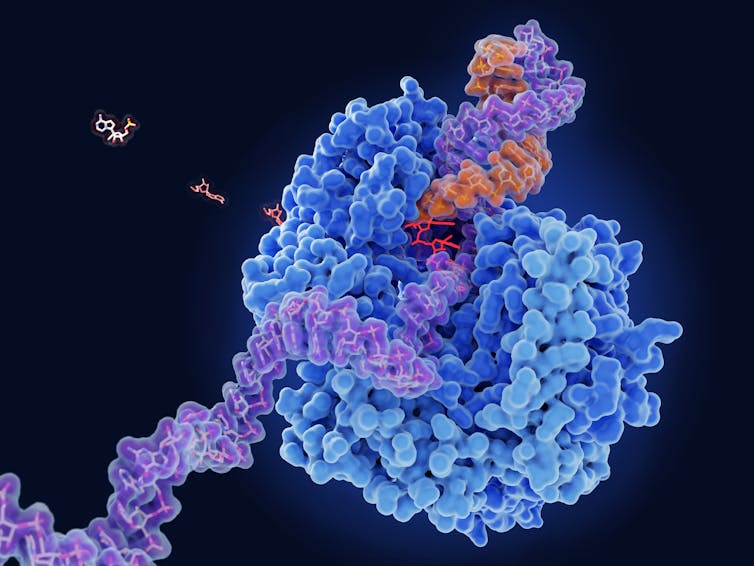
Moreover, although cells also have their own polymerases, they never replicate RNA like viruses do. This potentially allows chain-terminating antiviral drugs to selectively inhibit viral replication, reducing unwanted side effects.
Clearly, viperin does not fully protect against all RNA viruses – otherwise no RNA viruses would make you sick. It seems that some viral RNA polymerases, such as those in poliovirus, have evolved to discriminate against the antiviral molecules that viperin synthesizes and blunt their effect. However, viperin is only one arm of your immune system, which includes specialized cells and proteins that protect you from infection in other ways.
Ancient antivirals
Scientists discovered viperin about 20 years ago while searching for genes that turn on in response to viral infections. However, figuring out what viperin actually does proved very challenging.
Viperin’s function was particularly puzzling because it resembles an ancient group of proteins called radical SAM enzymes that are usually found in bacteria and molds. Notably, radical SAM enzymes are extremely rare in animals. Exposure to air rapidly inactivates them, and researchers thought they likely didn’t work in people. It’s still unclear how viperin avoids inactivation.
Researchers were clued in to viperin’s function when they noticed that the gene coding for viperin is next to a gene involved in synthesizing one of RNA’s building blocks. This observation led them to examine whether viperin might modify this RNA building block.
Following this discovery, researchers identified viperinlike proteins across all kingdoms of life, from ancient bacteria to modern plants and animals. This meant that viperin is a very ancient protein that evolved early in life, probably well before the advent of multicellular organisms – because even bacteria must fight viral infections.
As more complex life forms evolved, viperin was retained and integrated into the complex immune systems of modern animals. Thus, this most recently discovered arm of your immune system’s defenses against viruses is likely the most ancient.![]()
Neil Marsh, Professor of Chemistry and Biological Chemistry, University of Michigan
This article is republished from The Conversation under a Creative Commons license. Read the original article.