Alcohol and drugs rewire your brain by changing how your genes work – research is investigating how to counteract addiction’s effects

By Karla Kaun, Brown University
Many people are wired to seek and respond to rewards. Your brain interprets food as rewarding when you are hungry and water as rewarding when you are thirsty. But addictive substances like alcohol and drugs of abuse can overwhelm the natural reward pathways in your brain, resulting in intolerable cravings and reduced impulse control.
A popular misconception is that addiction is a result of low willpower. But an explosion of knowledge and technology in the field of molecular genetics has changed our basic understanding of addiction drastically over the past decade. The general consensus among scientists and health care professionals is that there is a strong neurobiological and genetic basis for addiction.
As a behavioral neurogeneticist leading a team investigating the molecular mechanisms of addiction, I combine neuroscience with genetics to understand how alcohol and drugs influence the brain. In the past decade, I have seen changes in our understanding of the molecular mechanisms of addiction, largely due to a better understanding of how genes are dynamically regulated in the brain. New ways of thinking about how addictions form have the potential to change how we approach treatment.
Alcohol and drugs affect brain gene activity
Each of your brain cells has your genetic code stored in long strands of DNA. For all that DNA to fit into a cell, it needs to be packed tightly. This is achieved by winding the DNA around “spools” of protein called histones. Areas where DNA is unwound contain active genes coding for proteins that serve important functions within the cell.
When gene activity changes, the proteins your cells produce also change. Such changes can range from a single neuronal connection in your brain to how you behave. This genetic choreography suggests that while your genes affect how your brain develops, which genes are turned on or off when you are learning new things is dynamic and adapts to suit your daily needs.
Recent data from animal models suggests that alcohol and drugs of abuse directly influence changes in gene expression in areas of the brain that help drive memory and reward responses.
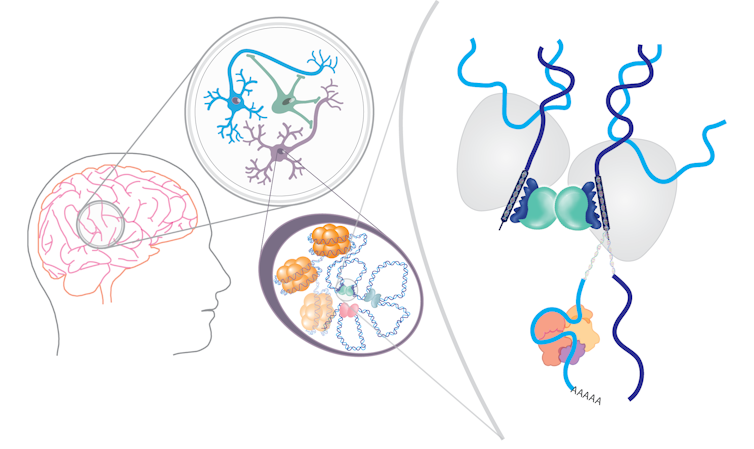
There are many ways addictive substances can change gene expression. They can alter which proteins bind to DNA to turn genes on and off and which segments of DNA are unwound. They can change the process of how DNA is read and translated into proteins, as well as alter the proteins that determine how cells use energy to function.
For example, alcohol can cause an alternative form of a gene to be expressed in the memory circuits in flies and people, resulting in changes in dopamine receptors and transcription factors involved in reward signaling and neuronal function. Similarly, cocaine can cause an alternative form of a gene to be expressed in the reward centers of mice, leading them to seek out more cocaine.
Exactly how these drugs cause changes in gene regulation is unknown. However, a direct link between alcohol consumption and changes in gene expression in mice provides a clue. A byproduct of alcohol being broken down in the liver called acetate can cross the blood-brain barrier and unwind DNA from histones in mouse memory circuits.
Alcohol, nicotine, cocaine and opioids also all activate important signaling pathways that are central regulators of metabolism. This suggests they can also affect many aspects of neuronal function and consequently affect which genes are expressed.
Changing brain gene activity with lifestyle
How addictive substances change cell function is complex. The version of a gene you’re born with can be modified in many ways before it becomes a functional protein, including exposure to alcohol and drugs. Rather than discouraging researchers, this complexity is empowering because it provides evidence that changes to gene expression in your brain aren’t permanent. They can also be altered by medications and lifestyle choices.
Many commonly prescribed medications for mental health disorders also affect gene expression. Antidepressants and mood stabilizers can change how DNA is modified and which genes are expressed. For example, a commonly prescribed drug for depression called escitalopram affects how tightly wound DNA is and can change the expression of genes important to brain plasticity.
Additionally, mRNA-based therapies can specifically change which genes are expressed to treat diseases like cancer. In the future, we may discover similar therapies for alcohol and substance use disorder. These treatments could potentially target important signaling pathways linked to addiction, altering how brain circuits function and how alcohol and drugs affect them.

Lifestyle choices can also affect gene expression in your brain, though researchers don’t yet know whether they can alter the changes induced by addictive substances.
Like alcohol and drugs, dietary changes can affect gene expression in many ways. In flies, a high sugar diet can reprogram the ability to taste sweetness by tapping into a gene expression network involved in development.
Intensive meditation, even after only one day, can also affect gene regulation in your brain through similar mechanisms. Attending a monthlong meditation retreat reduces the expression of genes that affect inflammation, and experienced meditators can reduce inflammatory genes after just one day of intensive mediation.
Work in animal models has also shown that exercise changes gene expression by altering both histones and the molecular tags directly attached to DNA. This increases the activity of genes important to the activity and plasticity of neurons, supporting the idea that exercise improves learning and memory and can decrease the risk of dementia.
From Dry January and beyond, many factors can have profound effects on your brain biology. Taking steps to reduce consumption of alcohol and drugs and picking up healthy lifestyle practices can help stabilize and bring long-lasting benefits for your physical and mental health.![]()
Karla Kaun, Associate Professor of Neuroscience, Brown University
This article is republished from The Conversation under a Creative Commons license. Read the original article.



